A guide to management practices at GTD Ireland:
Guidelines
The National Guidelines for the diagnosis, staging and Treatment of GTD are available at
Patient Registration
After you have been diagnosed with a “Molar Pregnancy” it is very important that your doctor registers your details with us so that we can monitor and take care of you in the best possible way. Your consent/agreement to this registration will be required.
The information collected about patients is stored electronically in a secure server at CUMH and is only available to specific staff who will treat you and work directly with the Gestational Trophoblastic Disease Registry. The GTD Registry is governed by the provisions of the Data Protection Act 1988 (Amended 2003).
Download Registration Form Here
If you are unable to register online you can email gtd@hse.ie
Diagnosis, Staging & Treatment of GTD
Clinician Guidelines
**The following documents are available to download at the end of this page**
GTD Registration Form
GTD Registration – Supplementary Notes
GTD National Clinical Guidelines
GTD – Suggested Guide – Initial Management of CHM
GTD – Suggested Guide – Initial Management of PHM
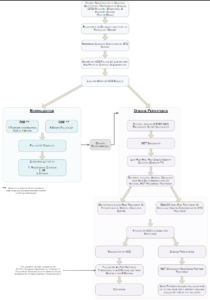
GTD Algorithm – Clinician Guidelines
The purpose of this website is to provide information to clinicians regarding the early management of molar pregnancies/trophoblastic disease and the registration of patients with the National Gestational Trophoblastic Disease (GTD) Treatment and Advisory Centre at CUMH. With the support of NCCP and the HSE we have published our national guidelines on the management of trophoblastic disease and these guidelines are in this section of the website. We have also included some “helpful hints” for clinicians regarding the initial management of patients with complete and partial moles and suggestions as to how these patients could be counselled regarding their diagnosis.
Our role at the GTD centre is to help monitor the follow up and make treatment decisions for patients with trophoblastic disease. Any patient that you register with us will be contacted by our clinical nurse specialist and the follow up protocol will be discussed. The registration form is available on this page for download. The registration form should be filled out in full and signed by the patient who consents to their data being stored on our database at our office in CUMH. The completed registration form should be emailed to our GTD office (gtd@hse.ie). The registration form will not be accepted without a copy of the histopathology report.
If you wish to ask advice about any patient with GTD or give us feedback on the website we are happy to receive an email at gtd@hse.ie
International Society for the Study of Trophoblastic Diseases (ISSTD)
An online edition of the book Gestational Trophoblastic Disease (3rd edition) is available on the International Society for the Study of Trophoblastic Diseases (ISSTD) website.
European Organisation for Treatment of Trophoblastic Disease (EOTTD)
The EOTTD is a European network for clinicians and researchers working in the field of GTD.
Research:
https://pubmed.ncbi.nlm.nih.gov/34172592/
https://pubmed.ncbi.nlm.nih.gov/32247260/
https://eottd.org/uploadedfiles/ann-oncol-2013-seckl-vi39-50.pdf
https://eottd.org/uploadedfiles/managementansprognosticfactorsofepithelioidtrophoblasctictumors.pdf

 GTD Registration Form
GTD Registration Form GTD Registration of Molar Pregnancy – Supplementary Notes
GTD Registration of Molar Pregnancy – Supplementary Notes